● From 1996 to 2015, the pharmaceutical industry in China experienced more than 30 overall forced price reductions in the past 20 years. Since then, the state has made efforts from all aspects of drug production, circulation and sales, and frequently introduced relevant policies to reduce drug prices.
China’s pharmaceutical market is divided into policy market and non-policy market. The former mainly includes public hospitals, and drugs are purchased by the government through unified bidding, which is greatly affected by the policy of reducing drug prices; The latter mainly includes pharmacies, clinics and private hospitals. Drug circulation is market-oriented and enterprises can set their own prices.
● The approval documents for the production of some APIs were monopolized by some enterprises, which led to the skyrocketing price of APIs. To solve this problem fundamentally, we should not only intensify the crackdown on the monopoly of APIs, but also improve the related approval system of APIs.
□ Our reporter Wen Lijuan
The retail price of a box of papaverine needles soared from 45 yuan to 399 yuan, a box of vitamin K1 needles rose from 99 yuan to 259 yuan, a box of tramadol tablets rose from 13.15 yuan to 30.3 yuan, a box of adrenaline needles rose from 29.1 yuan to 60.15 yuan, and a bottle of shark liver alcohol tablets rose from 33.75 yuan to 68.25 yuan … …
Recently, the "Legal Daily" reporter learned exclusively from a hospital in Hunan Province that many commonly used drugs for rescue, hemostasis and leukocytosis have increased their prices several times or even dozens of times since last year. At the same time, the prices of many commonly used drugs in the retail of pharmacies in the market have also risen sharply.
In recent years, China has successively introduced a series of measures such as canceling drug addition and "4+7" quantity procurement to promote drug price reduction. However, in the context of these measures, why do some commonly used drugs still have price increases or even supply cuts? What other aspects should we do to really let the people eat cheap drugs and rest assured drugs? The reporter conducted an investigation into this.
The purchase price of drugs has risen sharply.
The market price is climbing all the way.
Because of the rising drug price, Wang Yu (pseudonym), a native of Hunan Province, recently frequently asked people to buy cefixime from other places — — Her 4-year-old child often has respiratory infections, and this medicine has become a regular medicine at home. In the pharmacies near her home, the retail price of cefixime is around 15 yuan, while other city and county pharmacies only need three or four yuan.
Years of experience in buying medicines for children, and working in a hospital, Wang Yu found that the price increase of some commonly used drugs in clinic in recent two years was outrageous.
She gave the reporter a list of price increases: protamine, dexmedetomidine hydrochloride, cedilanid, allopurinol, dobutamine injection, calcium gluconate injection, rifampicin and some gout drugs. From last year to this year, there have been different price increases.
Take a box of dobutamine needles as an example. From August 2018 to December 2018, the retail price was 97.55 yuan; In January 2019, the retail price rose to 266 yuan; In April 2019, it rose to 460 yuan again.
Behind the rising retail price of drugs, the purchase price is also rising: since last year, the purchase price of a box of vitamin K1 needles has risen from 99 yuan to 259 yuan, a box of tramadol tablets has risen from 13.15 yuan to 25 yuan, a box of adrenaline needles has risen from 24 yuan to 51 yuan, and a bottle of shark liver alcohol tablets has risen from 28 yuan to 58 yuan.
In addition to the price increase of some commonly used drugs in clinic, there are also many categories of commonly used drugs in pharmacy retail.
"Baking soda tablets for treating hyperacidity, the price of 100 tablets in the first two years was only one or two dollars. These days, I was already in 11 yuan when I bought it again. " In this regard, Zhang Li (pseudonym), a practitioner in the pharmaceutical industry, feels incredible.
The reporter inquired about the drug price of 315.com and found that at present, the lowest price of baking soda tablets in 20 pharmacies in China is a bottle from 9.9 yuan, and the highest price is 17.8 yuan.
Li Hao (pseudonym), who is engaged in drug sales in Beijing, also has many feelings. Because the child’s body resistance is weak, he often goes to the drugstore to buy Icoxin (vitamin AD drops). However, in the second half of last year, this medicine was suddenly out of stock, and after the supply resumed in April this year, it actually rose by nearly 20 yuan. "In the past, a box cost more than 20 yuan, but now it costs 39.8 yuan." Li Hao said.
In addition to the price increase, what makes Li Hao feel helpless is that "it is often not available".
The reporter’s investigation found that, in fact, the drug out-of-stock mode mentioned by Li Hao is actually a common tactic used by many pharmaceutical companies to raise prices.
"If they want to raise prices, they usually announce that they are out of stock first, and then resume supply at intervals, so that they can raise prices." In the interview, the person in charge of a pharmacy named Chen in Shangrao City, Jiangxi Province told the reporter, "If they raise prices, I can only follow them, because everyone is rising."
According to the person in charge, since 2014, some drugs have experienced abnormal price increases. In the past year, more and more commonly used drugs have increased their prices, and they have become more and more crazy. "This year, nitroglycerin is the most prominent. The prices of nitroglycerin tablets (0.5 mg *100 tablets) and injections (10 ml) have risen from 4 yuan and 20 yuan last year to 55 yuan and 110 yuan respectively."
From May 24 to 26, the reporter visited nearly 20 pharmacies in Chaoyang District and Haidian District, and found that vitamin B complex rose from 1.5 yuan to 10 yuan, with an increase of 600%. Yan Teling rose from 3 yuan in 2015 to 9 yuan at the end of 2018, with an increase of 200%; Pain-relieving tablets rose from 2.5 yuan to 9 yuan, with an increase of 260%. Compared with the previous two years, the prices of chlorpheniramine, roxithromycin, Jiangya No.0, norfloxacin capsules, nasal drops, etc. all increased in different degrees.
In addition, Angong Niuhuang Pills, Chuanbei Pipa Syrup, Yunnan Baiyao Aerosol, Sangju Yinqiao Powder, Compound Berberine Tablets, Qingfei Huatan Pills, 999 Ganmaoling, Sanjiu Weitai, Huanglian Shangqing Tablets and other Chinese patent medicines also have price increases. However, the prices of some Chinese patent medicines have declined slightly in the near future.
Price reduction policies are frequently introduced.
Some drug prices have risen instead of falling.
What puzzles many patients is that on the one hand, the national policy of reducing drug prices is constantly introduced, on the other hand, the prices of many commonly used drugs are rising.
The reporter found out that in 1996, the State Planning Commission (later renamed as the National Development and Reform Commission) promulgated the Interim Measures for the Administration of Drug Prices, and regained the pricing power of drugs. By 2015, the National Development and Reform Commission, the State Health Planning Commission, the Ministry of Human Resources and Social Security and other seven departments jointly issued the Opinions on Promoting Drug Price Reform, stipulating that the original drug prices set by the government should be cancelled except narcotic drugs and psychotropic drugs of the first category. In the past 20 years, the pharmaceutical industry in China has experienced more than 30 times as a whole.
Since then, the state has made efforts from all aspects of drug production, circulation and sales, and frequently introduced relevant policies to reduce drug prices. In 2018 alone, a number of policies were introduced: in April, drugs that passed the consistency evaluation of generic drugs were required to be included in the procurement catalogue in time; In August, it was required to increase doctors’ medical treatment fees and medical service fees and reduce drug prices; At the end of 2018, the "4+7" quantity procurement was implemented, and the average price of 25 drugs that successfully entered the tender was reduced by 52%, with the highest drop of 96%.
Since various measures to reduce drug prices have been implemented one after another, why are some commonly used drugs still increasing in price or even cutting off supply?
Shi Lichen, founder of Beijing Dingchen Management Consulting Co., Ltd., a medical consulting organization, explained to reporters that China’s pharmaceutical market is divided into policy market and non-policy market. The former mainly includes public hospitals, and drugs are purchased by the government through unified bidding, which is greatly affected by the policy of reducing drug prices; The latter mainly includes pharmacies, clinics and private hospitals. Drug circulation is market-oriented and enterprises can set their own prices.
"The reasons for the price increase of some commonly used drugs need to be specifically analyzed from these two markets." Shi Lichen said: in the policy market, even if purchasing in quantity, there are still three factors affecting the process of reducing drug prices in the field of drug bidding. First, after the government bidding, it is difficult for some hospitals to guarantee the purchase quantity with pharmaceutical companies; Second, it is difficult to get the payment back in time, which affects the operation of pharmaceutical companies; Third, some local authorities are easy to "kick the ball", which makes it difficult for pharmaceutical companies to complain when they encounter the first two problems.
In the non-policy market, Shi Lichen believes that most of the time, the policy of reducing drug prices is difficult to produce obvious price-oriented effect, and eventually the prices of some commonly used drugs in this market rise due to comprehensive factors. Among them, the price increase of upstream APIs is an important reason.
Game between parties behind price increase
Raw materials are artificially monopolized.
The so-called raw material medicine is the effective component of medicine. After adding some auxiliary materials to the raw material medicine, it becomes the medicine bought by patients.
In Shi Lichen’s view, if the increase of APIs exceeds five times, it may be caused by artificial monopoly. "Monopoly is mostly due to problems in the circulation of APIs."
Shi Lichen’s judgment was verified by a person in charge of a pharmaceutical company in Anhui Province.
The head of this pharmaceutical company has been deeply involved in the field of pharmaceutical circulation for many years, and he knows the ropes well. He also bought out drugs from large pharmaceutical companies on behalf of the company.
He told reporters that many times, API merchants will collude with monopoly, hoard and sell to pharmaceutical factories at high prices. Even if API manufacturers are punished by the government for monopoly, they can’t fundamentally curb their monopoly behavior, because most of the raw materials are in the hands of a few raw material manufacturers. "The government’s crackdown has curbed their behavior of driving up drug prices, but it can’t control the number of their shipments. If a pharmaceutical factory needs 10 kilograms of raw materials, the raw material supplier deliberately supplies only 1 kilogram to it, resulting in a serious shortage of production in the pharmaceutical factory, and the finished drugs on the market will be scarce. "
In another case, a third-party commercial company signed a sales agreement with an API company to buy out all the raw materials and then sell them to several preparation factories, and the API company could not participate in the pricing. This behavior is called "underwriting" or "controlling sales" in the industry. Once it is "controlled", other pharmaceutical factories can’t buy raw materials, or they can only buy them at high prices, resulting in drug supply interruption or rising costs.
Wu Huifang, general manager of Beijing Dongfang Bite Technology Co., Ltd., once said in an interview with Caijing magazine that some API companies are willing to cooperate with underwriting companies in consideration of profits. For example, if the annual demand for a certain raw material medicine is 100 kilograms, the underwriting company will give an order of 80 kilograms to a raw material pharmaceutical factory, and all the raw materials of this factory will be sold to it, which can guarantee the profit, that is, the fixed sales and fixed production. Whether an API will be controlled or not depends on the output. Subject to the financial strength of the underwriting company, underwriting mainly occurs in small varieties of APIs. The raw materials that are underwritten are rare, with an annual output of hundreds of tons, generally below 50 tons or 60 tons, or even 10 tons or 20 tons, and few manufacturers can produce them normally.
The monopoly of API production will seriously restrict the preparation market. According to the sampling survey results of the corresponding proportion of APIs to preparation manufacturers, one API enterprise corresponds to 169 preparation enterprises at most.
According to Li Qing, deputy director of the Price Supervision Bureau of the National Development and Reform Commission, among the 1,500 chemical APIs in China, only one enterprise can produce 50 APIs, only two enterprises can produce 44 APIs and only three APIs can produce 40 APIs.
"Once the price of APIs rises or the supply is cut off, it will affect the production of downstream enterprises, resulting in a substantial increase in the production cost of drugs, which will be reflected in the price increase when it is transmitted to consumer terminals." Li Yong, an associate professor of the shortage drug research group of China Pharmaceutical University, told reporters.
In addition to the artificial monopoly leading to the increase in the price of APIs, the tightening of environmental protection policies and the normalization of drug quality and management control supervision are also the reasons.
The production of APIs belongs to manufacturing industry, and the upstream is chemical products, which will pollute water and air. The environmental protection control of such products is getting tighter. In order to meet the requirements of environmental protection, many pharmaceutical companies have invested in transformation, and some pharmaceutical companies are facing the situation of closure or merger.
The reporter inquired about the annual report of drug supervision statistics released by National Medical Products Administration in 2018 and 2015, and found that as of the end of November 2018, there were 4,441 manufacturers of raw materials and preparations nationwide; As of the end of November 2015, this figure was 5065.
The annual report explained that during the license renewal period of production enterprises, some enterprises did not pass GMP (good manufacturing practice) certification and temporarily did not have the conditions to renew their licenses. The direct consequence of the decrease in the number of pharmaceutical factories is that the fierce competition in the drug market has decreased, which has pushed up the price of drugs.
In addition, the new version of GSP (Good Quality Management Practice for Pharmaceutical Trading) requires strict verification of pharmaceutical production processes and strengthened supervision, which has increased the ticket-passing cost of commercial companies. "As an upstream manufacturer, we can only guarantee the interests of all links and ensure the smooth flow of product channels through a certain price increase." The relevant person in charge of a pharmaceutical factory in Hunan Province told the reporter.
Solve the skyrocketing drug prices
Urgent need to improve the examination and approval system
On April 3rd, Li Keqiang, Premier of the State Council of the People’s Republic of China emphasized at the the State Council executive meeting that it is normal for drug prices to fluctuate within a reasonable range, but if there is a sharp price increase, we must attach great importance to it. Especially for the commonly used first-aid drugs and rescue drugs that are urgently needed in clinic, once the supply is not guaranteed, it will threaten people’s lives and health, and patients’ lives will never be allowed to be traded. This kind of incipient problem must be resolutely curbed.
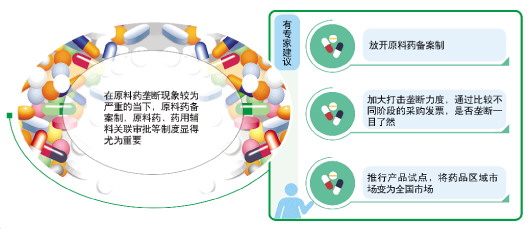
Tracing back to the source, in order to curb the price increase of some commonly used drugs, the monopoly of APIs can not be ignored. People in the industry interviewed by reporters generally believe that the transformation of APIs from approval system to filing system is imminent.
Zhang Buyong, general manager of Minenet, a medical data service platform, believes that this is actually the fact that the production approval documents of some APIs are monopolized by some enterprises, which leads to the skyrocketing price of APIs. To solve this problem fundamentally, we should not only intensify the crackdown on the monopoly of APIs, but also improve the related approval system of APIs.
Shi Lichen also told reporters that at the moment when the monopoly of raw materials is more serious, the system of filing raw materials, approval of raw materials and pharmaceutical excipients is particularly important.
"First, let go of the API filing system; Second, intensify efforts to crack down on monopoly. By comparing the purchase invoices at different stages, it is clear at a glance whether there is monopoly; Third, it is also beneficial for pharmaceutical companies to implement product pilots and turn the regional drug market into a national market. " Shi Lichen said, "Once these three points are implemented, the drug prices in the non-policy market will come down."
Li Yong also suggested that from a legal perspective, the focus should be on strengthening law enforcement, especially on the investigation and punishment of artificial monopoly of pharmaceutical raw materials, so as to eliminate the driving force for the abnormal rise of drugs from the source; In addition, relax the access threshold for the supply of pharmaceutical raw materials and introduce market competition mechanism; Reduce the intermediate circulation links from pharmaceutical raw materials to consumer terminals and reduce unnecessary costs; At the same time, establish an effective mechanism for the storage, monitoring, early warning and emergency response of commonly used drugs.
Cartography/Gao Yue